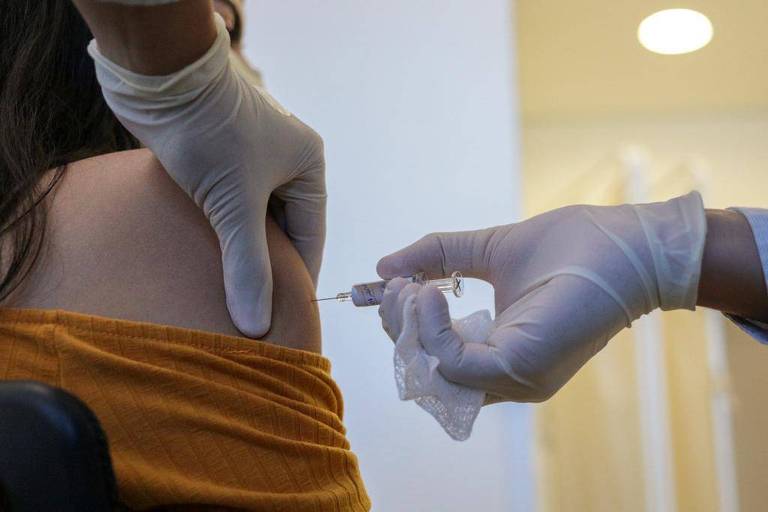
The National Health Surveillance Agency authorized on Wednesday (11) the resumption of testing of the Coronavac vaccine, produced by the Chinese company Sinovac in partnership with the Butantan Institute.
The tests had been suspended on Monday (9) after the agency reported that it had received notification of a serious adverse event in a volunteer. According to the agency, the objective was to verify safety and risk/benefit data.
According to the director of the Butantan Institute, Dimas Covas, the death of a volunteer was unrelated to the vaccine. The investigators' main suspicion is that the person, a 32-year-old chemist, has committed suicide or overdosed. The police report, obtained by Folha, records the case as "consummated suicide."
An opinion of an independent international committee received by Anvisa on Tuesday afternoon (10) also ruled out the relationship with the vaccine.
In a note, Anvisa said on Wednesday (11) that, "after evaluating the new data presented by the sponsor after the study was suspended, it understands that it has sufficient subsidies to allow the resumption of vaccination and continues to monitor the investigation of the outcome of the case so that the possible causal relationship between the unexpected EAG and the vaccine is defined".
Translated by Kiratiana Freelon